As the limitations of autologous cell therapy approaches become evident, many cell therapy innovators are making the switch to allogeneic strategies. But, what is the best starting material for allogeneic cell therapy? And how can you navigate all the challenges associated to this approach? In a new article, Stem Cell Reports analyzed the history and status of clinical studies using human pluripotent stem cells (PSCs).
A marked trend towards iPSC-based cell therapy
The Human Pluripotent Stem Cell Registry has observed a significant shift in the use of human PSCs as starting materials for cell therapies. The majority of studies up to 2017 utilized human embryonic stem cells. However, since 2018, the number of clinical studies using iPSC-based cell therapy has increased tremendously (Fig. 1). This shift in stem cell usage and the challenges it poses are key aspects of ongoing research in the field of cell therapies.
Figure 1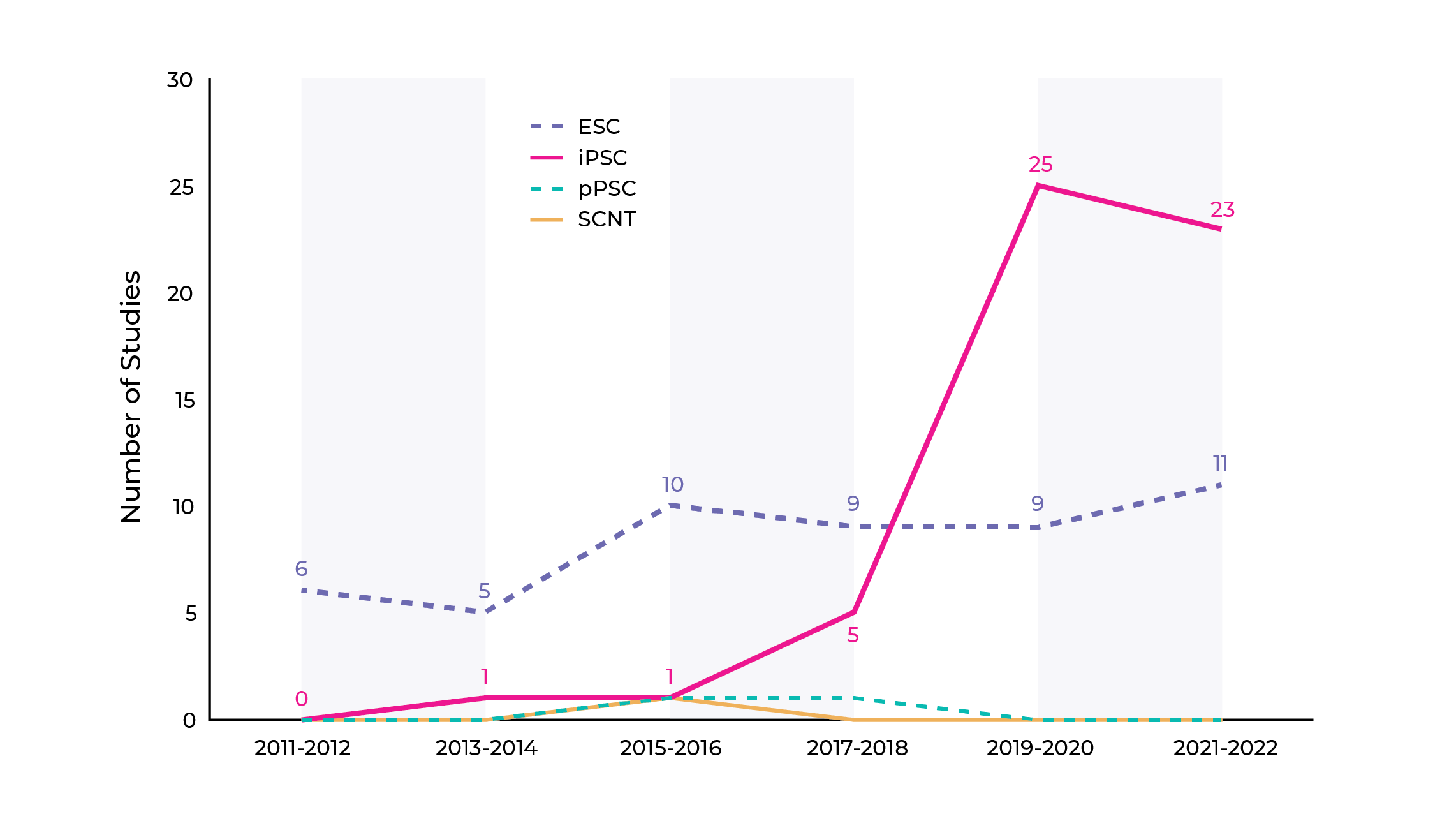
The use of iPSC in allogeneic cell therapy is a game-changing strategy with profound implications. These versatile cells can be genetically modified in a more efficient manner to enhance safety and efficacy. Although genetic engineering of iPSCs is challenging, they also have the potential to reduce cell variability and the costs associated with cell line development.
The scalability of iPSCs ensures a steady and standardized supply of cells, enabling broader accessibility to cutting-edge therapies for patients.
NK cells: one of the most popular PSC-derived cell types in clinical trials.
Over the past decade, the landscape of PSC-derived cell types used for therapeutic purposes has expanded from 3 to 22, targeting a total of 44 indications. Retinal pigment epithelial cell remain the main cell type but immune and cardiac cells have been gaining popularity in the past 5 years.
Notably, a surge in natural killer (NK) cell-like derivatives has emerged, with 18 studies initiated within the past 5 years, mainly in oncology. In this therapeutic area, engineered chimeric antigen receptor (CAR)-T cells are also widely studied and are showing remarkable value in targeting both liquid and solid tumours.
This diversity of cell types, each with its unique therapeutic potential, highlights the exciting and rapidly evolving landscape of cell-based therapies, particularly in the realm of immune cells.
iPSC-based cell therapy: a complex process with increased risk due to lack of transparency
Among the approaches to cell therapy development, iPSC-based allogeneic cell therapy stands out as one of the most intricate, yet promising approaches. The potential for developing a more scalable and accessible therapeutic against lower costs, make iPSCs a compelling choice. However, as an allogeneic treatment, immunogenicity and immune rejection remain a serious and realistic risk that must be mitigated to ensure the therapies’ safety and tolerability. Additionally, scale up, standardization and long-term efficacy are ongoing challenges.
In addition to that, the lack of transparency in clinical studies with PSC-derived cells is hampering reproducibility and regulatory approval. Critical information, such as the identity of the PSC lines used, characterization of PSC-derived cells or quality control protocols, is often not publicly available or inconsistently reported.
The absence of standardized reporting makes it difficult to compare data across studies and understand the causes of trial failures. Moreover, information on the end products is rarely accessible, impeding regulatory processes and increasing global costs.
A partnership can help you navigate cell therapy development and manufacturing
As the field progresses, it's crucial to establish an environment that encourages collaboration to overcome the many challenges of iPSC-based cell therapies. With the anticipated growth of clinical studies, more data will become accessible to be implemented in future research. Combined with consistent reporting and standardization of processes, this could accelerate the acceptability of advanced therapeutics.
For therapeutic developers, strategic collaborations with experienced CDMOs can significantly de-risk the development process by enhancing the reproducibility and reliability of the product and accelerating the translation of iPSC-based cell therapies into safe and effective treatments.
Source: Kobold, S., Bultjer, N., Stacey, G., Mueller, S. C., Kurtz, A., & Mah, N. (2023). History and current status of clinical studies using human pluripotent stem cells. Stem Cell Reports. https://doi.org/10.1016/j.stemcr.2023.03.005

