Reprogramming

You can either use our off the shelf available iPSC or we can reprogram from another donor of choice. We can provide data details on our iPSC lines upon request
Gene Editing

Operating in a highly automated environment for editing and clone selection, our team leverages precision CRISPR-mediated multiplex gene editing of iPSC cells to generate product-specific cell banks.
- >90% editing efficiencies using Cellistic’s nuclease of choice
- Editing of multiple target genes simultaneously, driving significant time savings
- Reduction of off-target integration through precise CRISPR-mediated, homology-directed repair
- Quality control through generation of homogenous MCB establishes genomic product integrity
- Manufacturing that begins at the MCB can be confirmed to be free of genetic aberrations
Master Cell Banking

MCBs and working cell banks (WCBs) generated through the Pulse Platform deliver precisely the quality, consistency, and reliability that innovative immunotherapy development demands.
- State of the art GMP facility to support manufacturing of your MCB using established processes
- Thorough cell bank characterization to ensure regulatory readiness under a GMP quality system
- Best-in-class culture conditions for the highest quality cells
- Inhouse cryopreservation capacity for thousands of vials
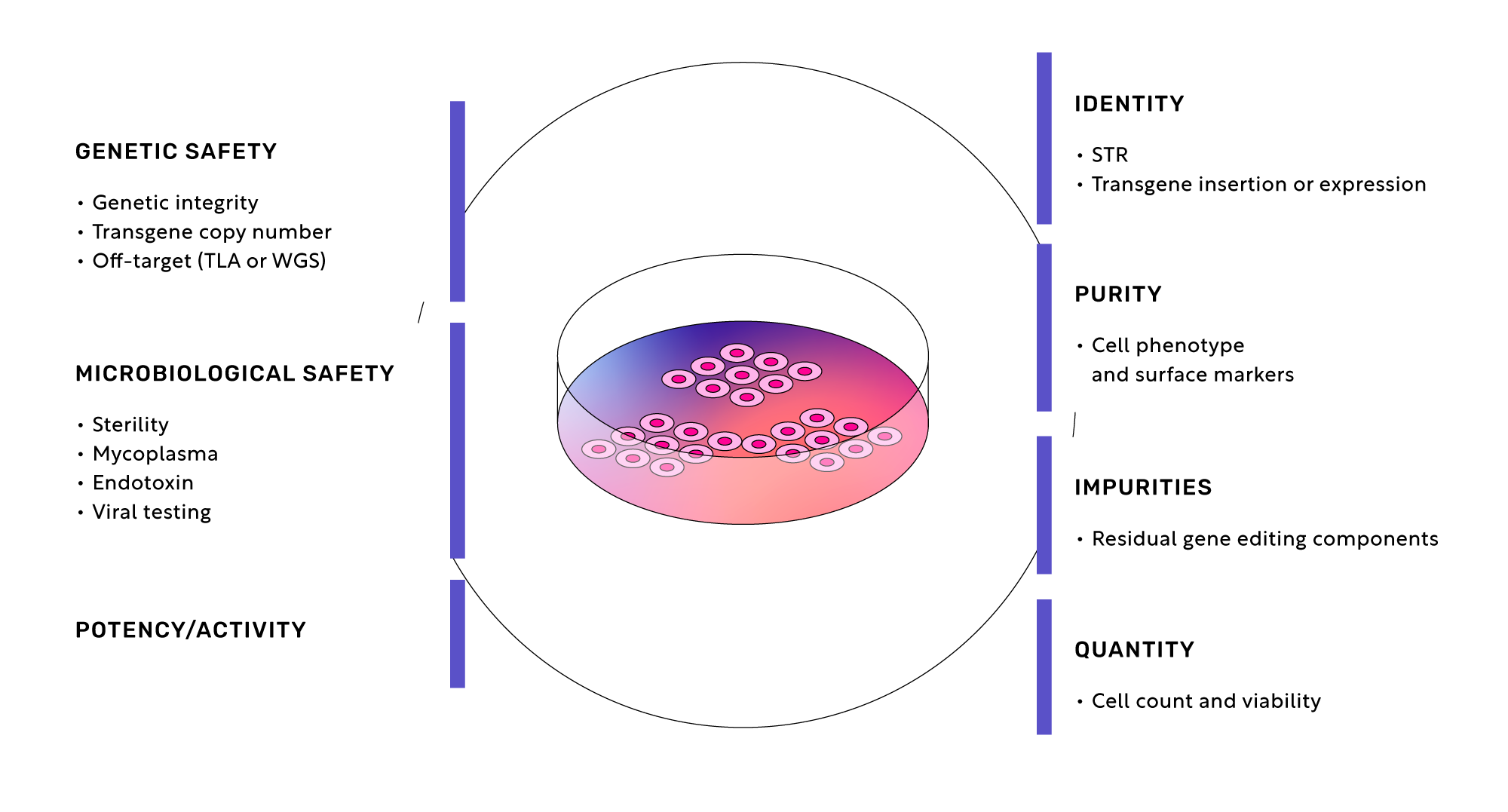
Quality Control

With the Pulse Platform, multiple clones with the desired genotype will be fully characterized after cryopreservation to enable extensive testing across seven key dimensions.

More on the iPSC science that gives Pulse its tempo
Download our latest poster, that provides insight into how we have generated immune-cloaked “off-the-shelf” allogeneic cell therapies.

Highly sound, highly efficient cell line development
Our Pulse Platform can help enable our partners to address a key challenge: time. Our value is demonstrated most acutely by helping clients decrease cell line development timelines. At Cellistic, we have refined and optimized development protocols over the last 24 months, representing activities and time you do not need to repeat and hence can shortcut your development timeline.
Purpose-built
CLD labs
Delivering state-of-the-art technology for cell reprogramming and proprietary STAR-CRISPR technology for multiplex gene editing, and mono-clonality assurance using UP.SIGHT
Comprehensive Quality Control
Using a process that delivers a homogenous product with high viability and strong cell recovery which is fully characterized for GMP master cell bank (MCB) manufacturing
Dedicated Quality System
Leveraging GMP principles in an iPSC-specific approach to risk management that enable efficient and precise reprogramming, editing, clone selection, and characterization
